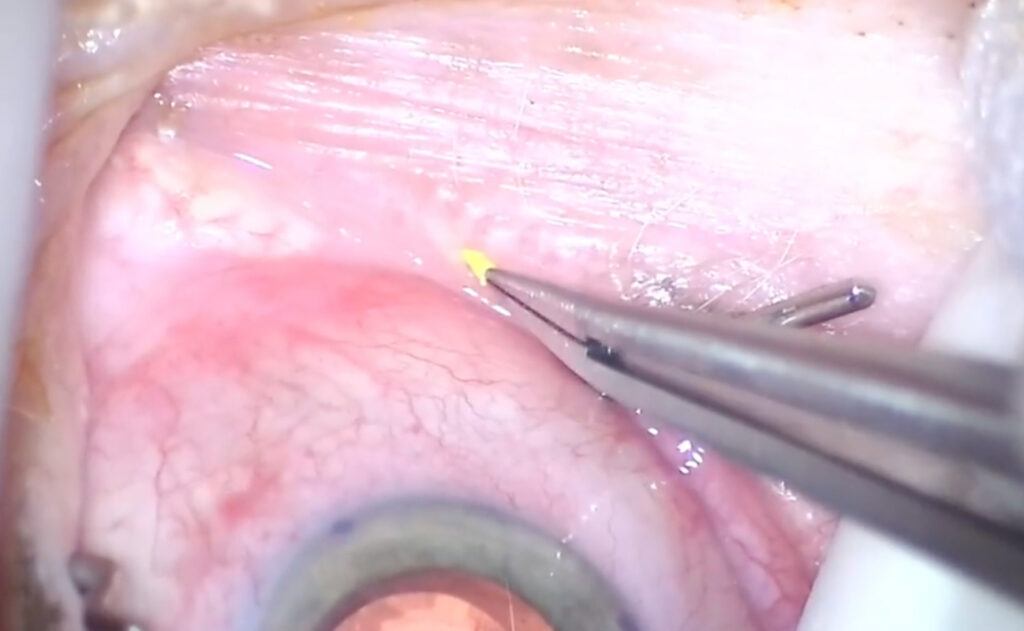
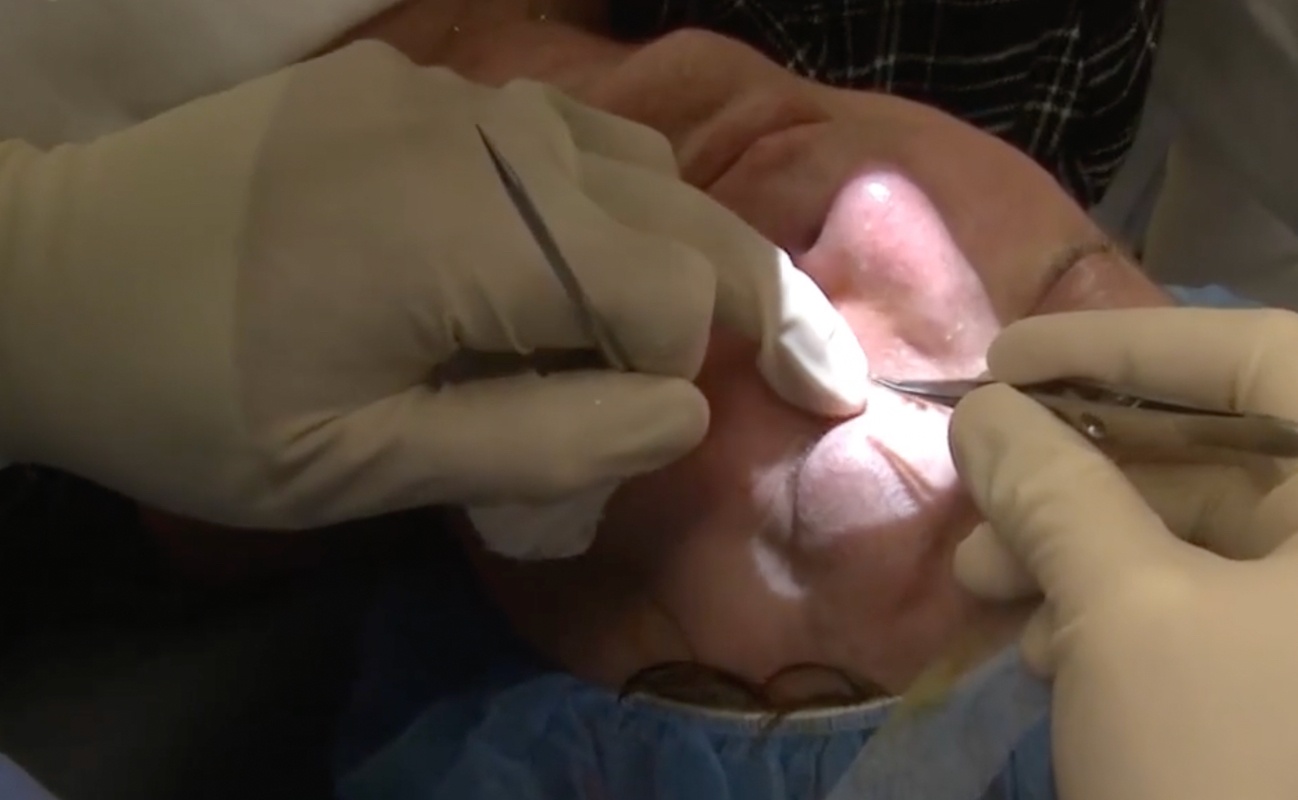
DEXTENZA
Why DEXTENZA?
A hands-free advancement in ophthalmic steroid treatment.1,2
Easy-to-insert* and preservative-free intracanalicular DEXTENZA offers patients a satisfying†
post-op experience—providing up to 30 days of sustained steroid coverage.1-5
INDICATIONS
DEXTENZA is a corticosteroid indicated for:
- The treatment of ocular inflammation and pain following ophthalmic surgery.
- The treatment of ocular itching associated with allergic conjunctivitis.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
DEXTENZA is contraindicated in patients with active corneal, conjunctival or canalicular infections, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella; mycobacterial infections; fungal diseases of the eye, and dacryocystitis.
Click here or see below for Important Safety Information.
*73.6% of physicians in Study 1, 76.4% in Study 2, and 79.6% in Study 3, for the treatment of ocular inflammation and pain following ophthalmic surgery, rated DEXTENZA as easy-to-insert.3,5
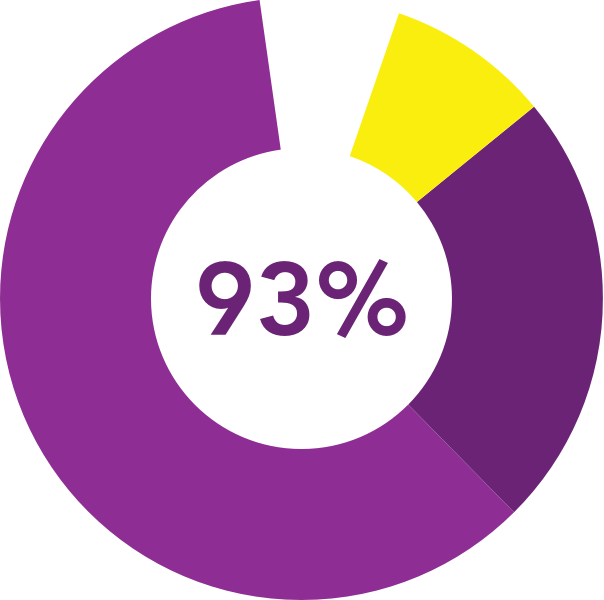
†93% (187/201) DEXTENZA patients were satisfied with the insert in the third Phase 3 Study for the treatment of ocular inflammation and pain following ophthalmic surgery.4

Features & Benefits of DEXTENZA

*73.6% of physicians in Study 1, 76.4% in Study 2, and 79.6% in Study 3, for the treatment of ocular inflammation and pain following ophthalmic surgery, rated DEXTENZA as easy-to-insert.3,5
How DEXTENZA Works
DEXTENZA is dexamethasone delivered via ELUTYX™ technology1,3,5
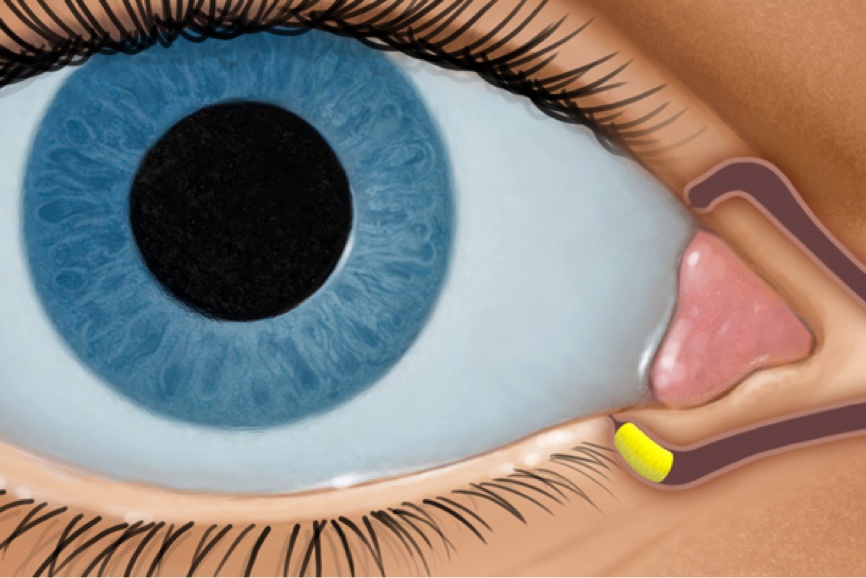
ACTIVATES1,3
- With moisture
- Swells to fit securely in the canaliculus
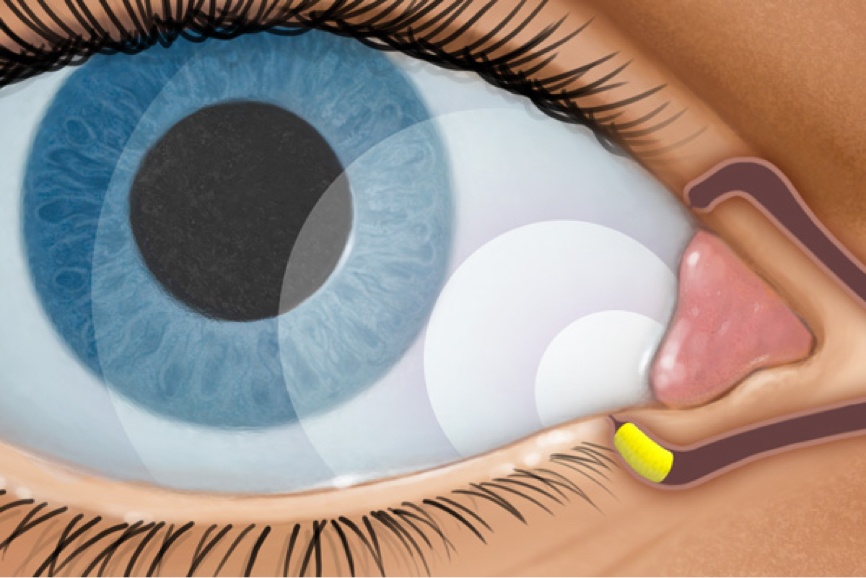
RELEASES1,3
- Dexamethasone for up to 30 days
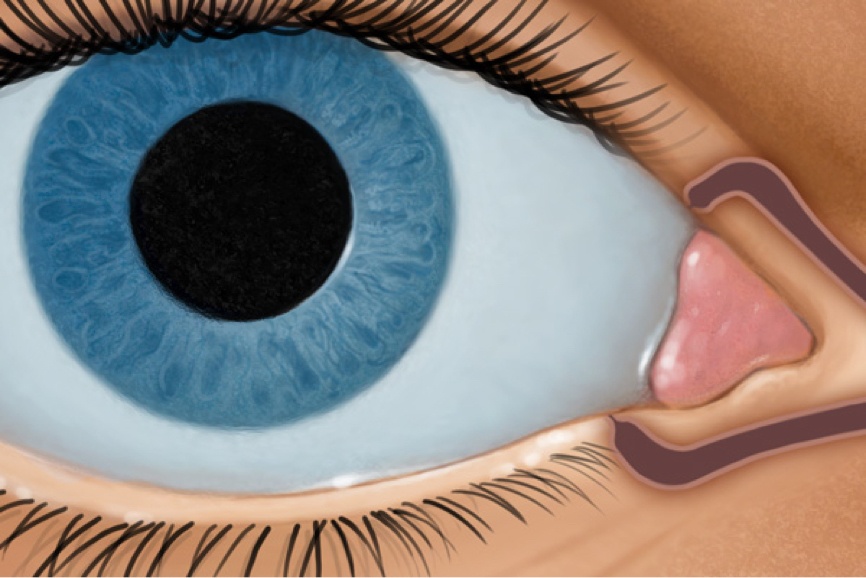
RESORBS1,3
- Slowly through the course of treatment
- Clears via the nasolacrimal duct
DEXTENZA In Use
Patient Experience With DEXTENZA Postoperatively
Patients reported highly favorable outcomes in comfort, convenience, & satisfaction4
Survey results based on the responses from 201 DEXTENZA patients who participated in the third Phase 3 Study for inflammation and pain following ophthalmic surgery.*
Study limitations6
- Qualitative written survey (Questions not validated)
- Relied on recall in an elderly population
**Intent-to-treat population.
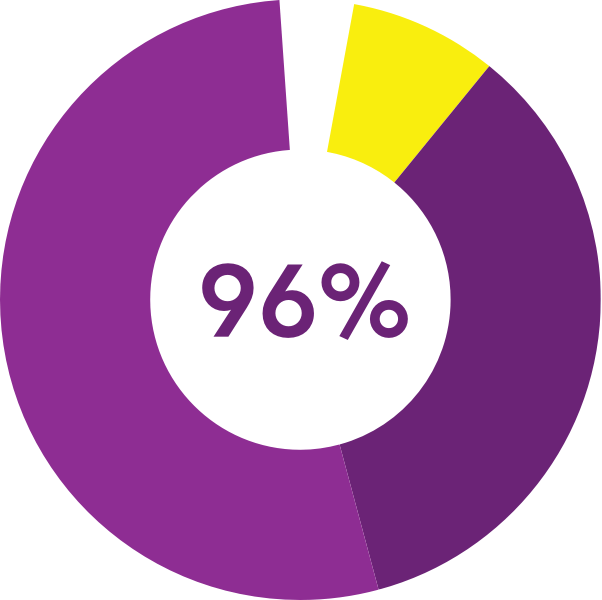
described the insert as
comfortable
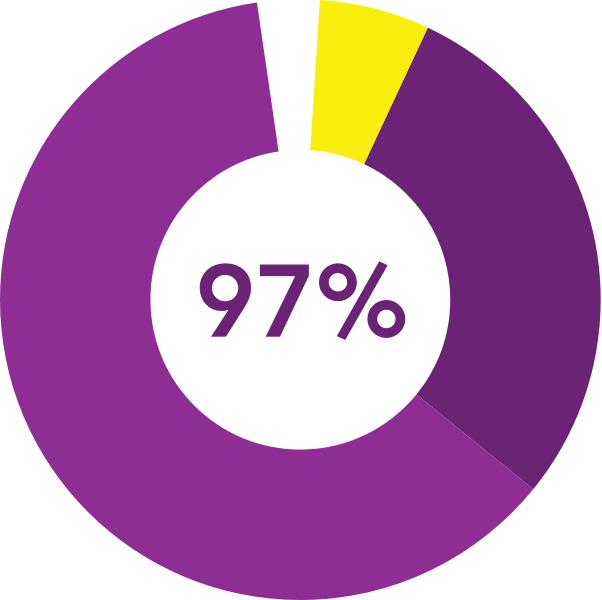
described the insert as
convenient
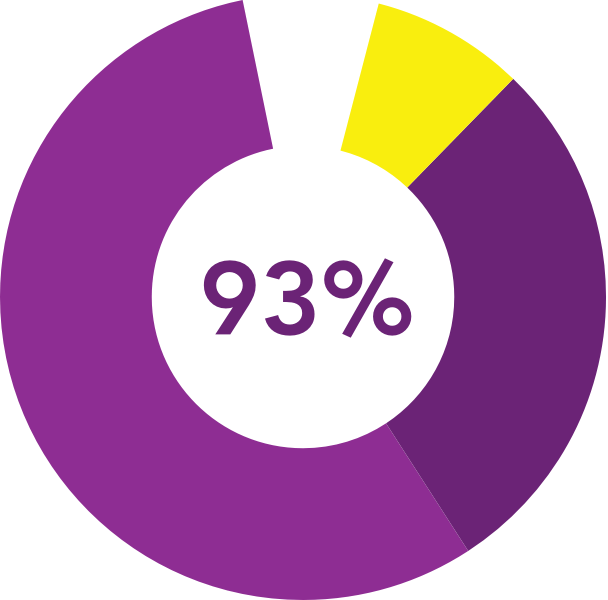
were satisfied
with the insert
were likely to request
the insert for future treatment
Interested In Learning More?
Important Safety Information
Contraindications
DEXTENZA is contraindicated in patients with active corneal, conjunctival or canalicular infections, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella; mycobacterial infections; fungal diseases of the eye, and dacryocystitis.
Warnings And Precautions
Intraocular Pressure Increase:
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. Intraocular pressure should be monitored during treatment.
Bacterial Infections:
Corticosteroids may suppress the host response and thus increase the hazard for secondary ocular infections. In acute purulent conditions, steroids may mask infection and enhance existing infection.
Viral Infections:
Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
Fungal Infections:
Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use. Fungal culture should be taken when appropriate.
Delayed Healing:
Use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation.
Other Potential Corticosteroid Complications:
The initial prescription and renewal of the medication order of DEXTENZA should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated.
Adverse Reactions
Ocular Inflammation and Pain Following Ophthalmic Surgery:
The most common ocular adverse reactions that occurred in patients treated with DEXTENZA were: anterior chamber inflammation including iritis and iridocyclitis (10%), intraocular pressure increased (6%), visual acuity reduced (2%), cystoid macular edema (1%), corneal edema (1%), eye pain (1%), and conjunctival hyperemia (1%). The most common non-ocular adverse reaction was headache (1%).
Itching Associated with Allergic Conjunctivitis:
The most common ocular adverse reactions that occurred in patients treated with DEXTENZA were: intraocular pressure increased (3%), lacrimation increased (1%), eye discharge (1%), and visual acuity reduced (1%). The most common non-ocular adverse reaction was headache (1%).
REFERENCES: 1. DEXTENZA [package insert]. Bedford, MA: Ocular Therapeutix, Inc; 2021. 2. Sawhney AS, et al., Inventors, Incept, LLC, Assignee. Drug delivery through hydrogel plugs. US Patent 8,409,606 B2. April 2, 2013. 3. Tyson SL, et al. J Cataract Refract Surg. 2019;45(2):204-212 [erratum in: 2019;45(6):895]. 4. Data on File 00837. Ocular Therapeutix, Inc. 5. Walters T, et al. J Clin Exp Ophthalmol. 2016;7(4):1-11. 6. Noecker RJ, et al. Evaluating the physician and patient experience of a dexamethasone insert (0.4mg) in patients having cataract surgery. Presented at: American Society of Cataract and Refractive Surgery Annual Meeting. April 16, 2018; Washington, DC.