
OTX-CSI
Sustained-Release Product Candidate For Dry Eye Disease
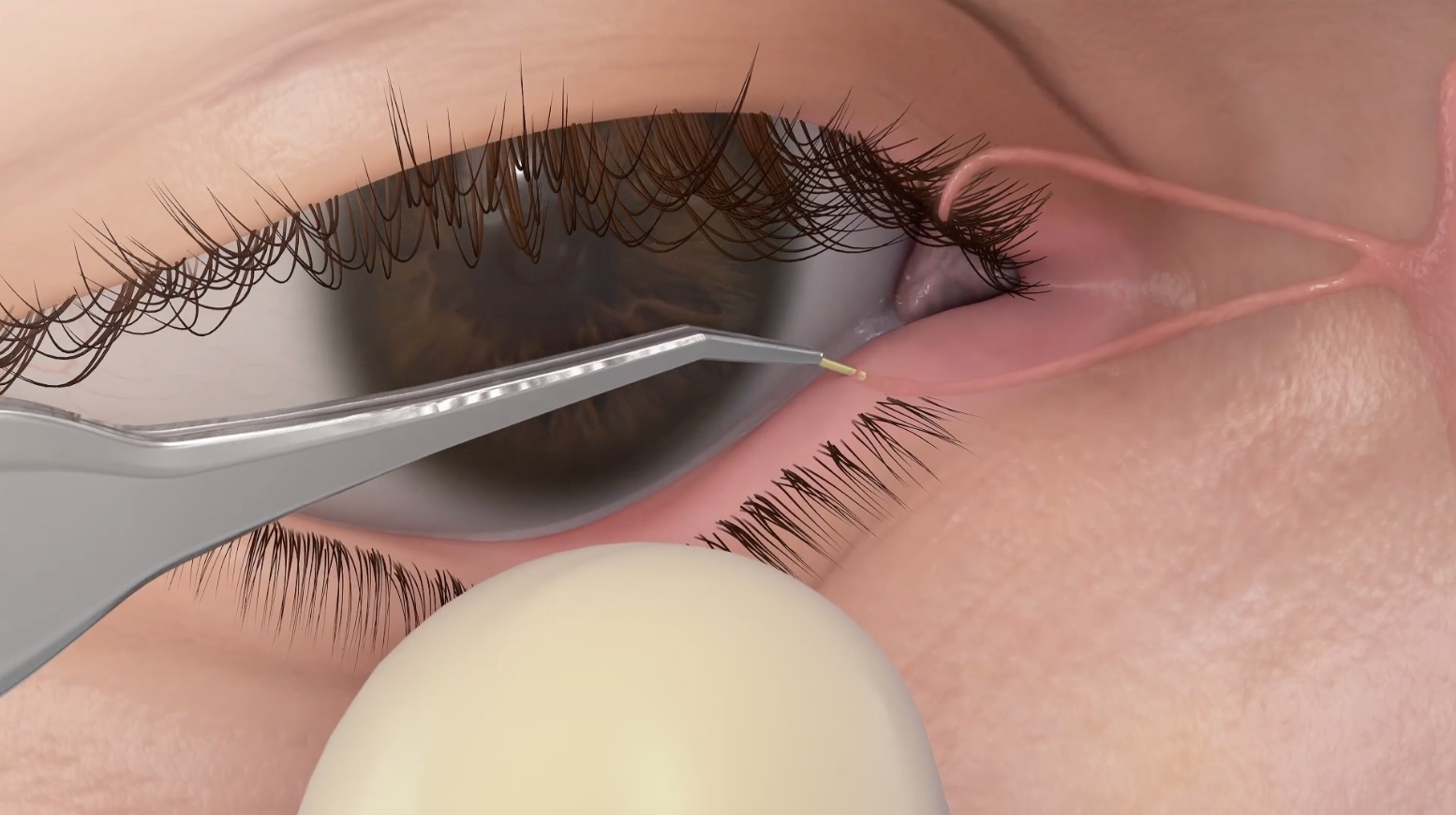
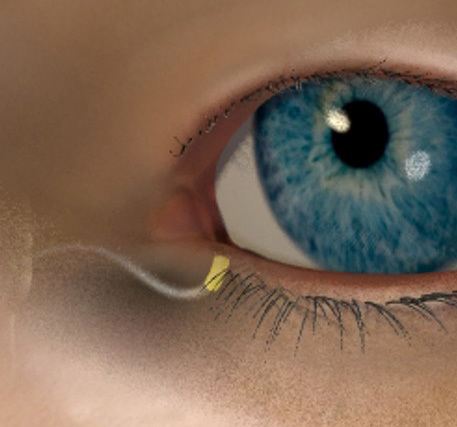
OTX-CSI is a long-acting, preservative-free cyclosporine intracanalicular insert being evaluated for the treatment of dry eye disease.1
Existing Treatments
- Slow onset of action for therapy2,3
- High level of burning and stinging upon administration2,4
- Burden of patient administration2,4
Product Candidate Attributes5
- Cyclosporine loaded in ELUTYXTM Technology
- Preservative-free
- Designed to deliver therapy up to 12 weeks with a single insert
- Occludes the punctum
- Fully biodegradable insert
Caution: OTX-DED is currently undergoing clinical evaluation and is limited by law to investigational use only. This product has not been approved by the FDA as safe or effective.
About Chronic Dry Eye Disease
Dry eye disease is a common condition with a global prevalence between 5% and 34% and totaling more than an estimated 16 million Americans.6,7 It is a chronic inflammatory disease that can be initiated by numerous extrinsic or intrinsic factors that lead to an unstable and hyperosmolar tear film.8,9 Known risk factors include aging, female gender, refractive surgery and autoimmune disease.9 According to the International Dry Eye Workshop, dry eye disease is defined as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”8 A primary mechanism responsible for dry eye is tear hyperosmolarity leading to tissue damage.8 Eye pain is caused by tear hyperosmolarity and loss of lubrication.8 Visual changes are due to tear and ocular surface abnormalities.8 Depending on severity, dry eye disease can limit daily activities such as using a computer, reading, driving and also adversely affect sleep and work productivity.10-12
REFERENCES: 1. Vanslette A. Evaluating Safety and Pharmacokinetics of OTX-CSI, a Sustained Cyclosporine Releasing Intracanalicular Insert in Beagles. Presented at the ARVO Annual Meeting; May 3-7, 2020; Baltimore, MD. 2. White DE, et al. Clin Ophthalmol. 2020;14:875-883. 3. Trattler W, et al. Clin Ther. 2006;28(11):1848-1856. 4. RESTASIS® (cyclosporine ophthalmic emulsion) 0.05% [prescribing information]. Irvine, CA: Allergan, Inc.; 2012. 5. Data on file 018. Ocular Therapeutix, Inc. 6. Messmer EM. Dtsch Arztebl Int. 2015;112:71-81. 7. Farrand KF, et al. Am J Ophthalmol. 2017;182:90-98. 8. Bron AJ, et al. Ocul Surf. 2017;15:438-510. 9. Stapleton F, et al. Ocul Surf. 2017;15:334-365. 10. Walker PM, et al. Cornea. 2010;29(6):607-612. 11. Kawashima M, et al. Clin Ophthalmol. 2016;10:1015-1021. 12. Yamada M, et al. Clinicoecon Outcomes Res. 2012;4:307-312.