We have the expertise and resources to redefine the retinal disease market

SIGNIFICANT MARKET OPPORTUNITY
Retinal disease is one of the leading causes of blindness1,2
1.8 million people are living with wet age-related macular degeneration (AMD) and 6.3 million with diabetic retinopathy (DR) in the U.S.3
Incremental improvements in durability have delivered substantial market opportunity
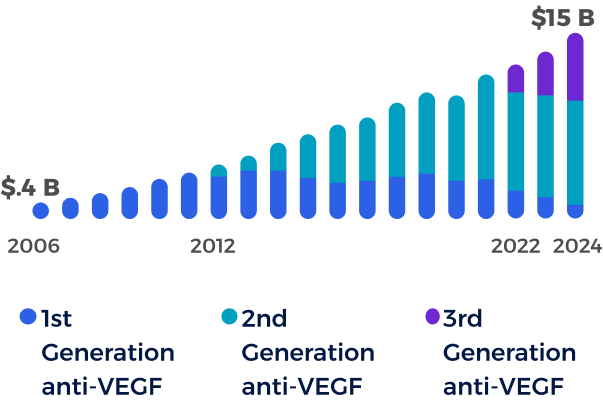
The global branded anti-VEGF market for retinal disease has grown from $0.4 B to $15 B in 20244

Significant need for more durable wet AMD treatment remains
90% of patients currently require injections every 1-3 months5
40% of patients discontinue treatment in the first year due to this heavy treatment burden6
Frequent injections may result in retinal fluid fluctuations and decreased adherence, which are associated with poor long-term outcomes7


Aiming to redefine treatment
AXPAXLI™ (also known as OTX-TKI), an investigational axitinib hydrogel administered by intravitreal injection, combines a highly potent and selective tyrosine kinase inhibitor (TKI) with our proven proprietary hydrogel. Phase 1 data provided a compelling foundation to advance toward Phase 3, with the goal to dramatically improve durability and long-term outcomes for patients with wet AMD and DR, and the potential to impact other retinal disease.8-12
AXPAXLI
An injection comprised of axitinib in a single hydrogel has the potential to provide continuous and consistent drug delivery for up to 12 months11,18
Nothing contained herein should be considered a solicitation, promotion or advertisement for any drug including the ones under development on this website.
All investigational product candidates are currently undergoing clinical evaluation. This content is not intended to convey any conclusion of safety or efficacy, and there is no guarantee that any product candidate will successfully complete development or gain FDA approval or other regulatory authority approval.
Redefining development
durability and improve long-term outcomes for patients

AXPAXLI’s potential in wet AMD:The SOL registrational trials are designed to showcase durability, repeatability, and flexibility
The SOL-X open-label extension trial evaluates long-term safety and potential disease-modifying impact
 | AXPAXLI’s potential in wet AMD: The SOL registrational trials are designed to showcase durability, repeatability, and flexibility The SOL-X open-label extension trial evaluates long-term safety and potential disease-modifying impact |

Superiority Trial

Non-Inferiority Trial
Patient selection
Randomization after anti-VEGF loading
Randomization after anti-VEGF loading
Trial design
Enable Q12M dosing on label
Enable superiority claim on label
Enable Q6M dosing on label
Show efficacy compared to standard of care
FDA alignment
Special Protocol Assessment (SPA) agreement
Type C, other written responses
Superiority Trial

Non-Inferiority Trial
Patient selection
Randomization after anti-VEGF loading
Randomization after anti-VEGF loading
Trial design
Enable Q12M dosing on label
Enable superiority claim on label
Enable Q6M dosing on label
Shows efficacy compared to standard of care
FDA alignment
Special Protocol Assessment (SPA) agreement
Type C written response

Open-Label Extension Trial
Patient selection
Patients who have completed two-year follow-up in either SOL-1 or SOL-R
Trial design
Three-year follow up with AXPAXLI Q6M dosing to assess long-term outcomes

AXPAXLI’s potential in DR:The complementary HELIOS registrational trials leverage a novel ordinal endpoint and are designed to unlock the diabetic market and support a broad DR label
 | AXPAXLI’s potential in DR: The complementary HELIOS registrational trials leverage a novel ordinal endpoint and are designed to unlock the diabetic market and support a broad DR label |

Superiority Trial

Superiority Trial
Patient selection
Moderate to severe non-proliferative diabetic retinopathy without center-involved diabetic macular edema
Moderate to severe non-proliferative diabetic retinopathy without center-involved diabetic macular edema
Trial design
Enable Q12M dosing on label
Enable superiority claim on label
Enable Q6M and Q12M dosing on label
Enable superiority claim on label
FDA alignment
Special Protocol Assessment (SPA) agreement
Superiority Trial

Superiority Trial
Patient selection
Moderate to severe non-proliferative diabetic retinopathy without center-involved diabetic macular edema
Trial design
Enable Q6M and Q12M dosing on label
Enable superiority claim on label
FDA alignment
Special Protocol Assessment (SPA) agreement
Redefining outcomes
disease population of 9.6 million in the U.S. alone3
ONGOING WET AMD Registrational Trials
1.8M
Patients with Wet Age-Related Macular Degeneration3


ongoing dr registrational trials
2.8M
Patients with Moderate-Severe Non-Proliferative Diabetic Retinopathy3
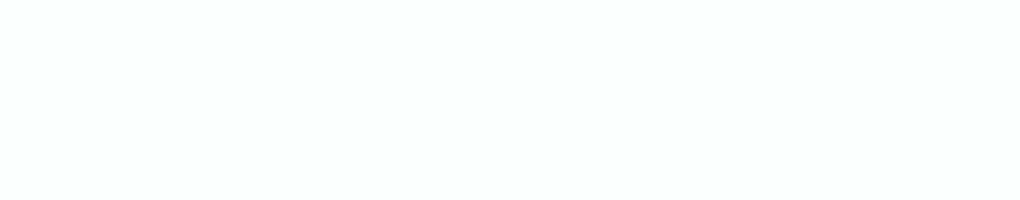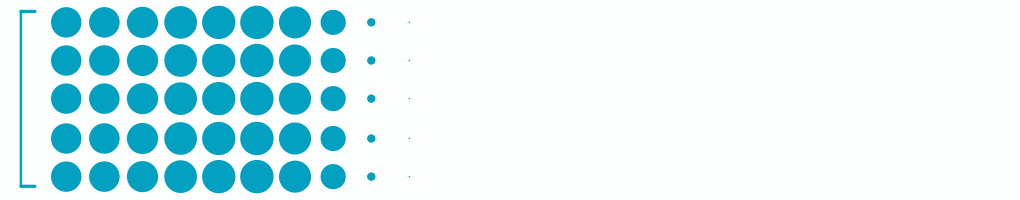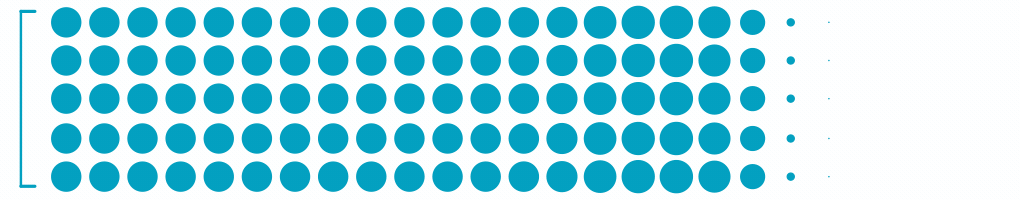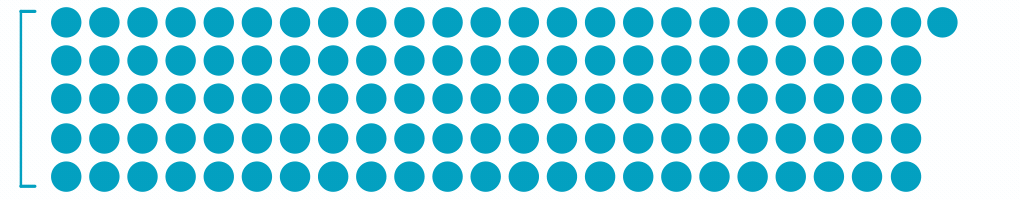

1.7M
Patients with Diabetic Macular Edema3
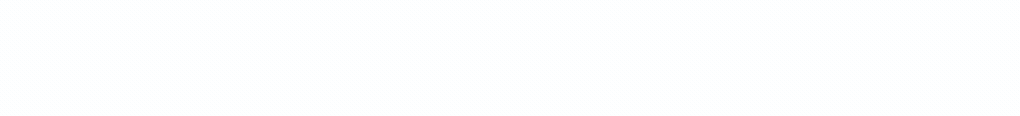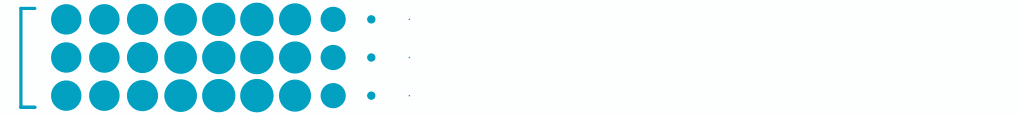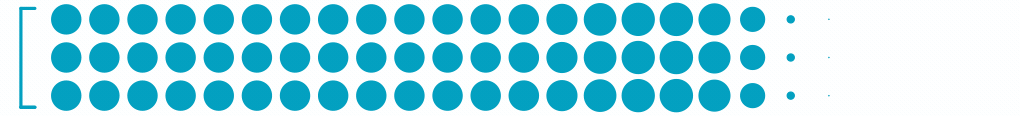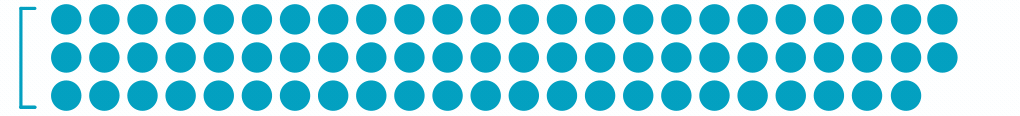

1.8M


FUTURE OPPORTUNITIES
1.5M
Patients with Retinal Vein Occlusion3


Each dot represents ~24,000 patients
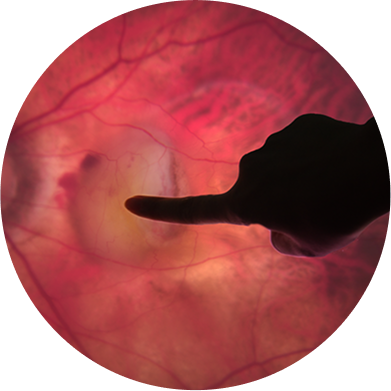
RETINA EXPERIENCE
Our team has unmatched expertise and an impressive track record of innovation in retinal disease
clinical trials designed or led by our team
combined years of treating patients with retinal disease
treatment launches across retinal disease
Stay informed
References
1. Wang W, et al. Int J Mol Sci. 2018;19(6):1816. 2. Di Carlo E, et al. J Clin Med. 2021;10(15):3297. 3. Market Scope. 2024 Retinal Pharmaceuticals Market Report: Wet AMD and Other Exudative Diseases. St. Louis, MO: Market Scope, LLC. 2024. 4. Data on file 01892. Anti-VEGF Revenue. Ocular Therapeutix, Inc. 5. Market Scope. Ophthalmic Market Trends: Quarterly US Retina Edition. St. Louis, MO: Market Scope, LLC. 2025. 6. Khanani AM, et al. Ophthalmology Retina. 2020;4(2):122-133. 7. Evans RN, et al. JAMA Ophthalmol. 2020;138(10):1109. 8. Zhao Y, et al. Oncologist. 2015;20(6):660-673. 9. Gross-Goupil M, et al. Clin Med Insights Oncol. 2013;7:269-277. 10. Liang C, et al. Mol Ther Oncolytics. 2022;24:577-584. 11. Blizzard CD, Inventors, Ocular Therapeutix, Inc., Assignee. Ocular implant containing a tyrosine kinase inhibitor. US Patent 11,439,592 B2. September 13, 2022. 12. Tyson SL, et al. J Cataract Refract Surg. 2019;45(2):204-212. 13. Sawhney AS, et al., Inventors, Incept, LLC, Assignee. Drug delivery through hydrogel plugs. US Patent 8,409,606 B2. April 2, 2013. 14. Blizzard C, et al. Clin Ophthalmol. 2021:15 2055–2061. 15. Boyer DS, et al. Evaluating Safety, Tolerability and Biological Activity of OTX-TKI, a Hydrogel-Based, Sustained-Release Intravitreal Axitinib Implant, in Subjects with Neovascular Age-Related Macular Degeneration. Presented at: American Academy of Ophthalmology Annual Meeting; November 13-15, 2020; Virtual. 16. Goldstein MH, et al. Invest Ophthalmol Vis Sci. 2020;61(7):4266. 17. McGrath M, et al. Invest Ophthalmol Vis Sci. 2014;55:472. 18. Data on file DOF 030. Ocular Therapeutix, Inc.