Clinical Trials
Ocular's team is aiming to redefine drug development

REDEFINING DEVELOPMENT
We’ve designed our complementary wet age-related macular degeneration (AMD) registrational trials
The SOL registrational trials are designed to show durability, repeatability, and flexibility

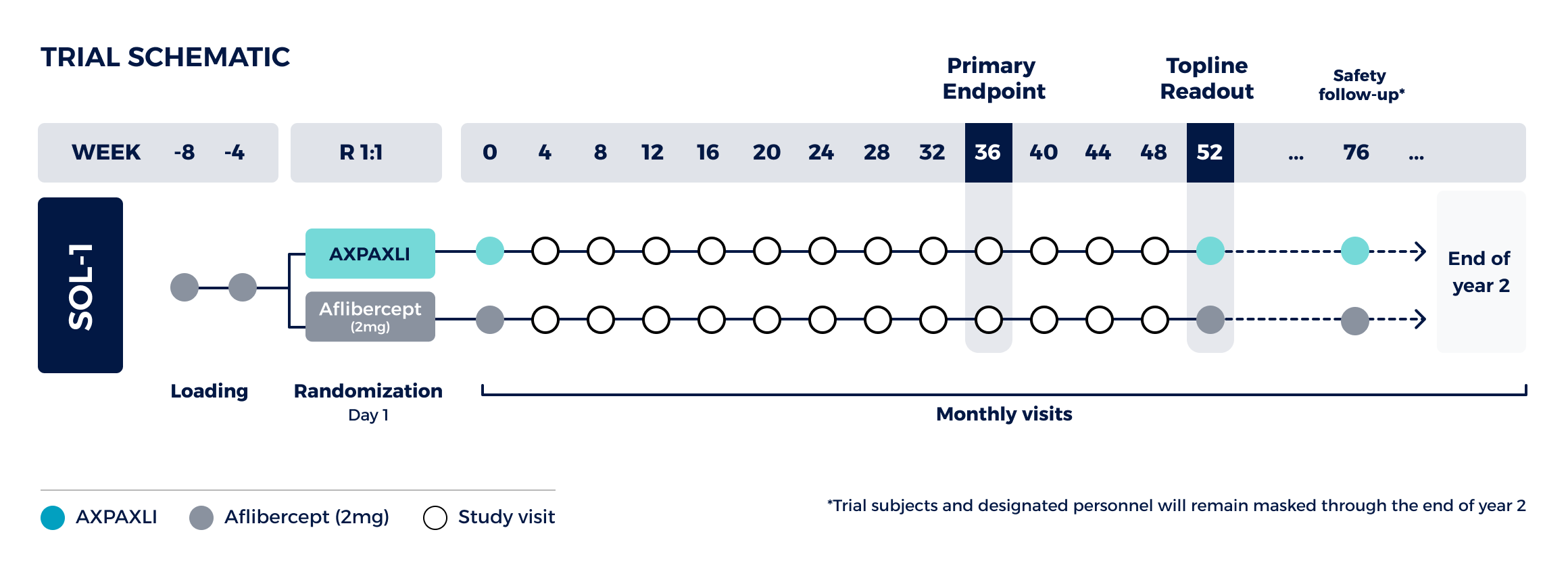
Superiority study comparing a single AXPAXLI™ (also known as OTX-TKI), an investigational axitinib hydrogel administered by intravitreal injection, dose to a single aflibercept (2mg) dose at Week 36
Design:
Two-arm trial with ~300 total subjects randomized 1:1
Primary Endpoint (week 36):
Demonstrate that a single AXPAXLI dose is superior to a single aflibercept 2mg dose based on proportion of subjects who maintained visual acuity, defined as <15 ETDRS (Early Treatment Diabetic Retinopathy Study) letters of BCVA (Best-Corrected Visual Acuity) loss from baseline

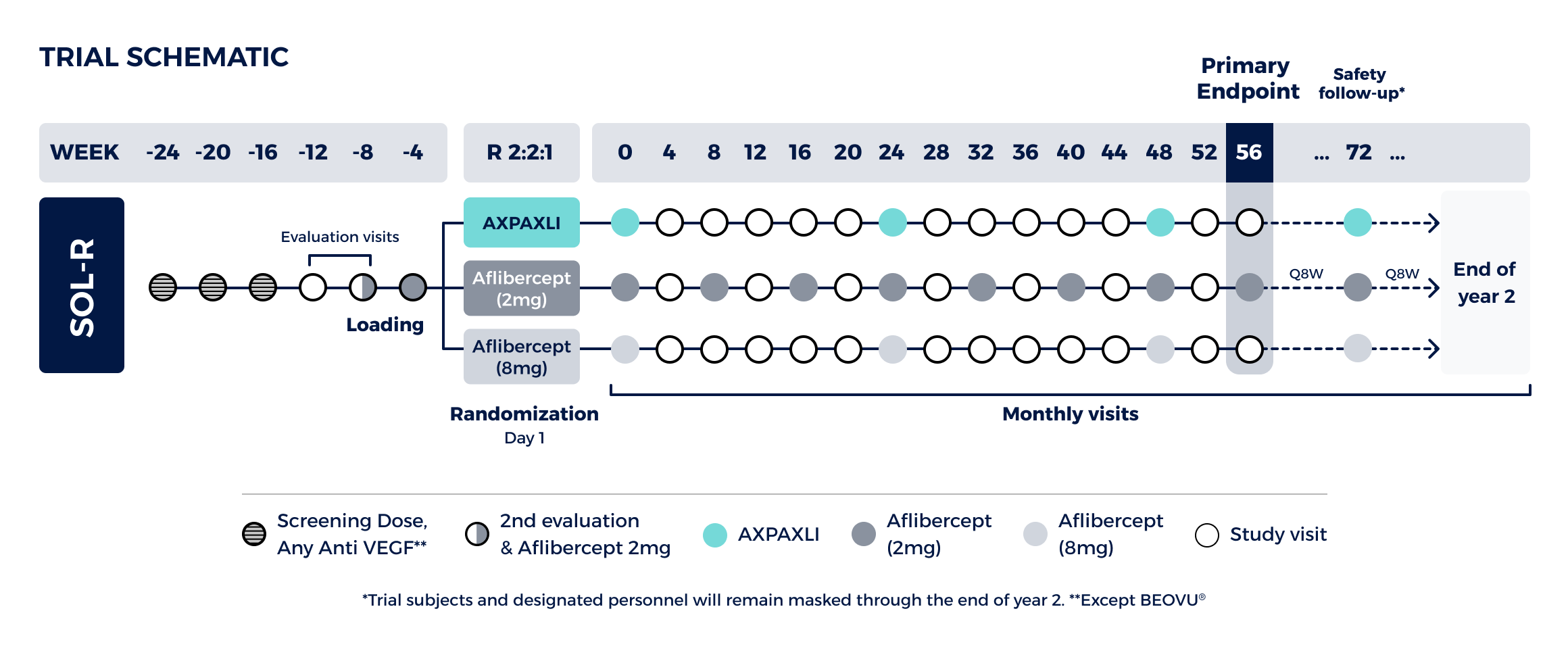
Non-inferiority study comparing AXPAXLI Q24W to aflibercept (2mg) Q8W at Week 56
Design:
Three-arm trial with 555 total subjects randomized 2:2:1
Primary Endpoint (week 56):
Demonstrate that AXPAXLI is non-inferior to fixed-dose aflibercept 2mg Q8W with respect to mean change from baseline in BCVA (Best-Corrected Visual Acuity) Week 56
Non-inferiority margin for the lower bound is -4.5 letters in BCVA at Week 56
Nothing contained herein should be considered a solicitation, promotion, or advertisement for any drug including the ones under development on this website.
All investigational product candidates are currently undergoing clinical evaluation. This content is not intended to convey any conclusion of safety or efficacy, and there is no guarantee that any product candidate will successfully complete development or gain FDA approval or other regulatory authority approval.
