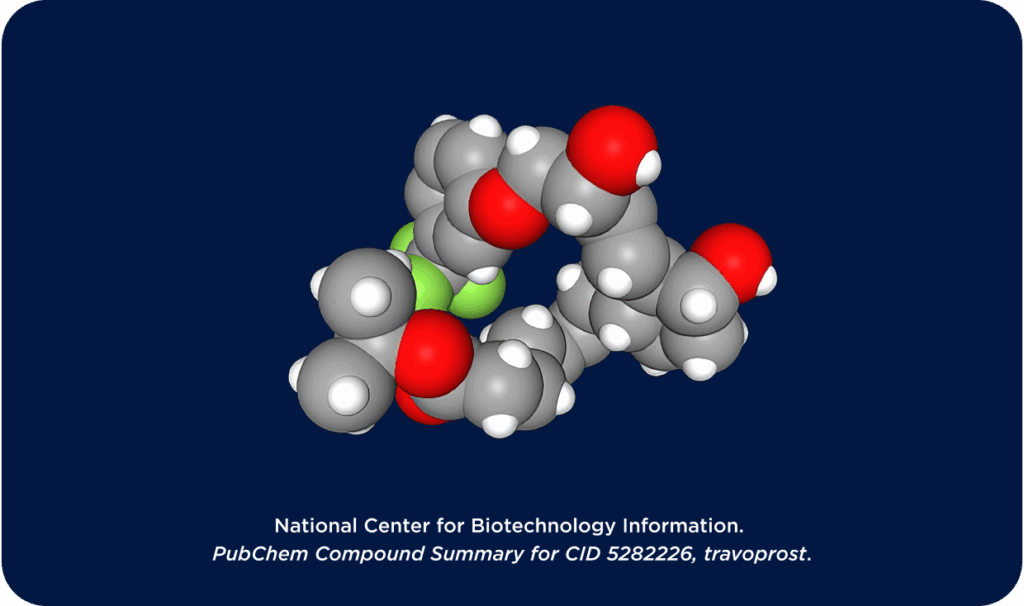
OTX-TIC
We aspire to redefine treatment for glaucoma
DISEASE BURDEN
Glaucoma is one of the leading causes of irreversible blindness worldwide1
3M Americans and 80M people worldwide live with glaucoma1
Glaucoma is a disease that damages the eye’s optic nerve. It occurs when fluid builds up in the front part of the eye, producing increased intraocular pressure (IOP). Elevated IOP is associated with damage to the optic nerve, which may result in irreversible vision loss.2
TREATMENT BURDEN
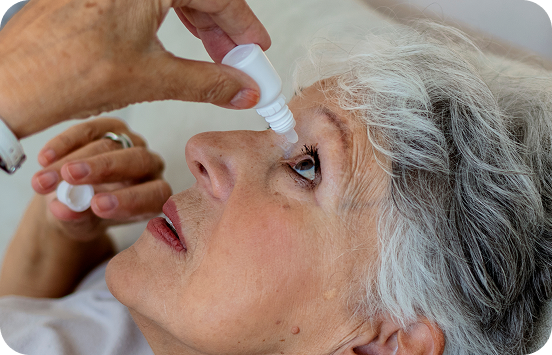
Treatment burden may lead to poor adherence which is associated with disease progression and vision loss
To lower IOP, initial treatment for glaucoma typically involves the long-term, daily administration of eye drops that may contain preservatives.3 This treatment regimen is often challenging for patients to maintain and results in poor patient satisfaction and reduced adherence.4,5 Poor adherence has been shown to be associated with disease progression and vision loss.6,7
OTX-TIC
We have the potential to redefine treatment for glaucoma
OTX-TIC (travoprost intracameral hydrogel) is an investigational injectable product which is intended to have an extended duration of action aiming for long-term reduction and maintenance of IOP to improve glaucoma patient outcomes. OTX-TIC is currently in Phase 2 clinical trials.
Nothing contained herein should be considered a solicitation, promotion, or advertisement for any drug including the ones under development on this website.
All investigational product candidates are currently undergoing clinical evaluation. This content is not intended to convey any conclusion of safety or efficacy, and there is no guarantee that any product candidate will successfully complete development or gain FDA approval or other regulatory authority approval.